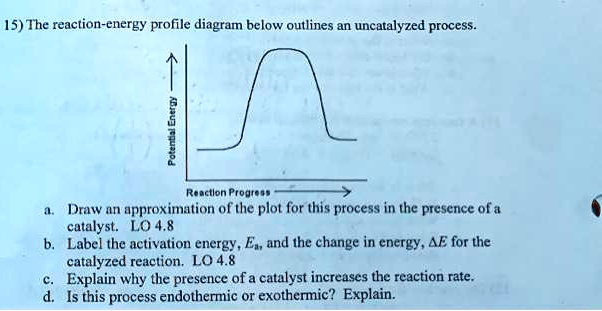
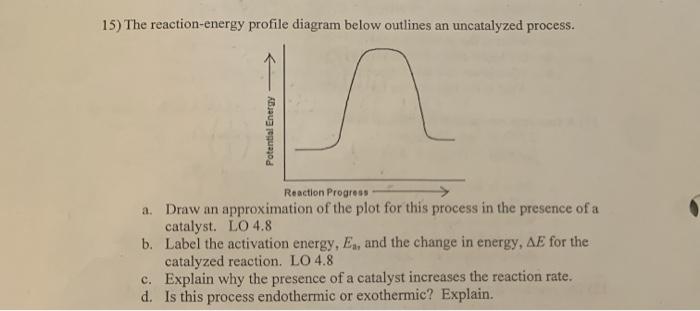
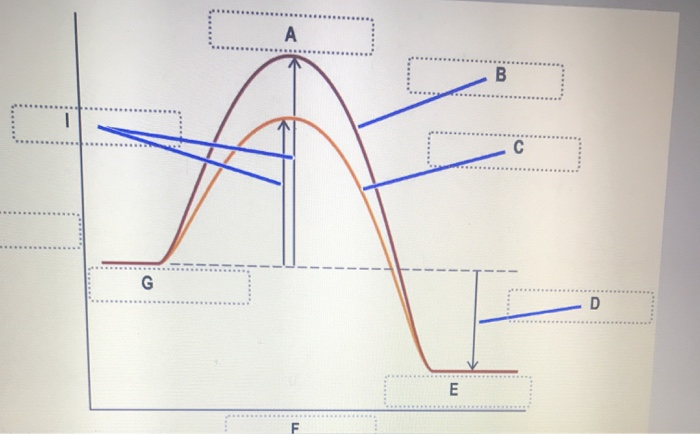
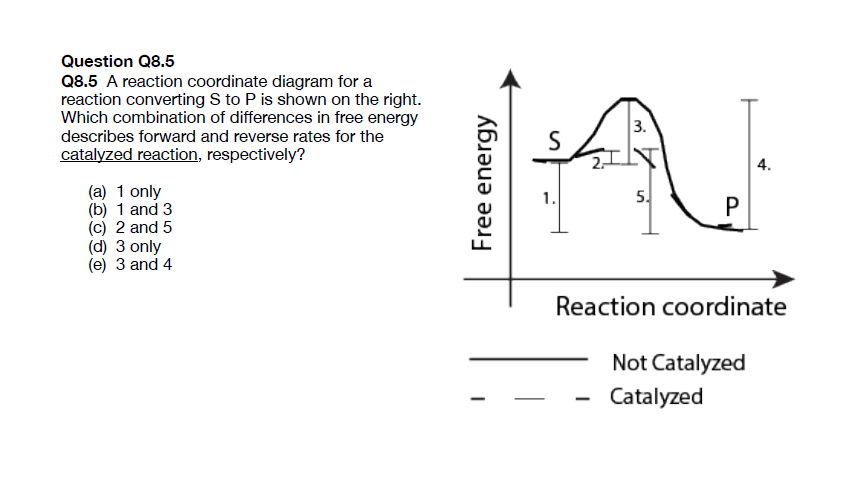
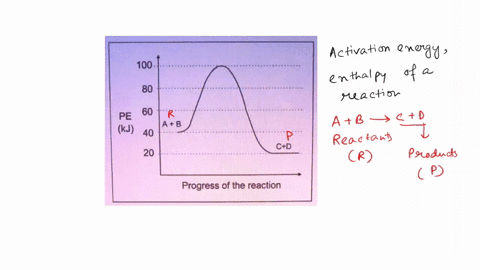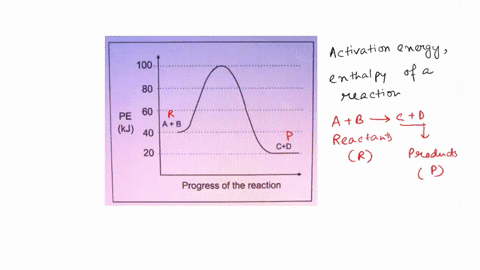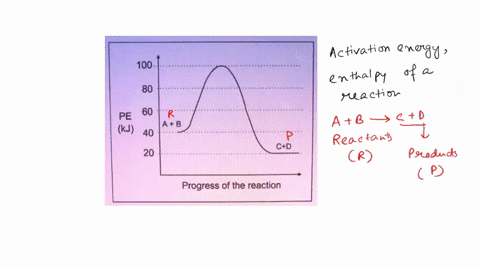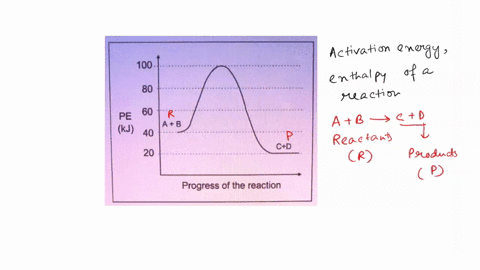
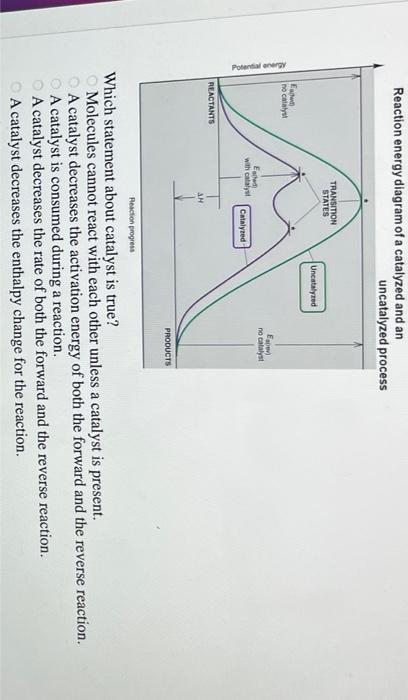
45 label the following reaction energy diagram for a catalyzed and an uncatalyzed process.
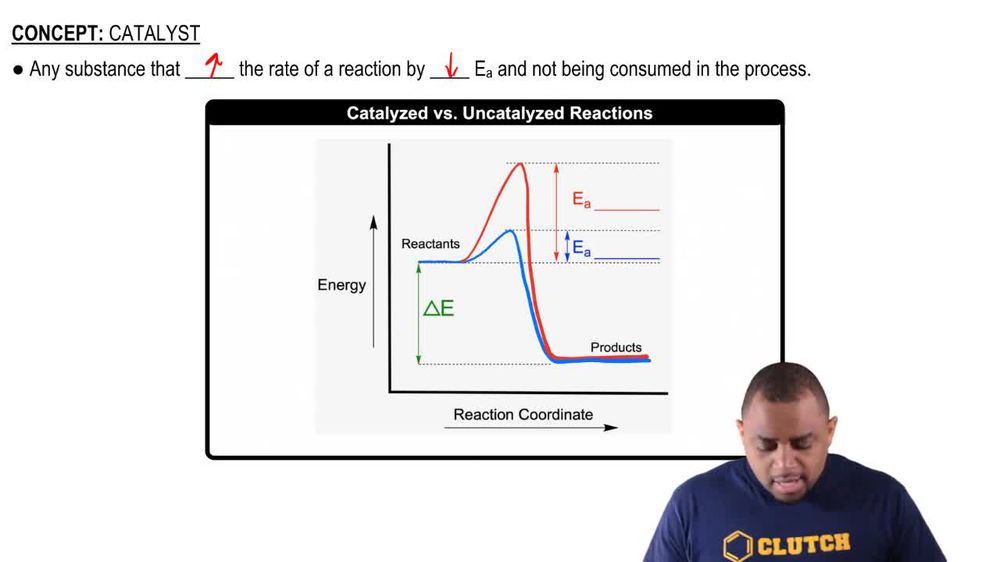
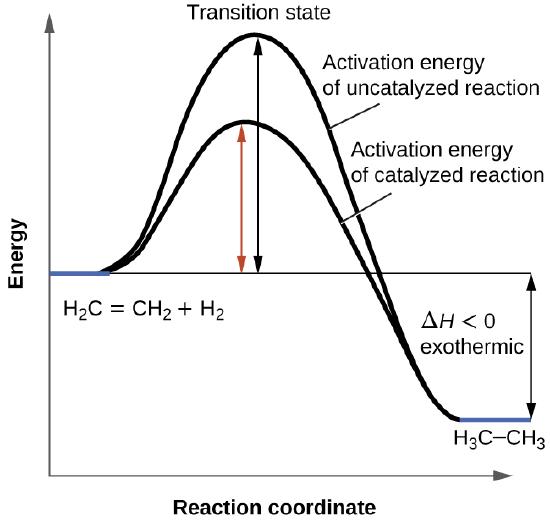
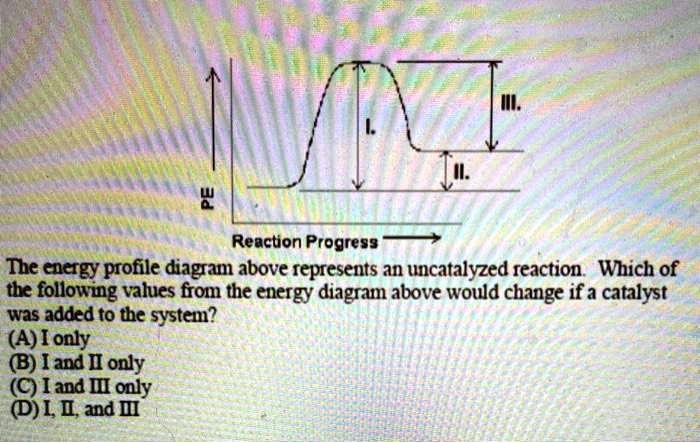
Free energy | Endergonic vs exergonic reactions (article) - Khan Academy Exergonic reactions are also called spontaneous reactions, because they can occur without the addition of energy. Reactions with a positive ∆ G (∆ G > 0), on the other hand, require an input of energy and are called endergonic reactions. In this case, the products, or final state, have more free energy than the reactants, or initial state. Types of catalysts (article) | Kinetics | Khan Academy Notice that the energies of the reactants and products are the same for the catalyzed and uncatalyzed reaction. Therefore, the overall energy released during the reaction, Δ H rxn \Delta \text H_{\text{rxn}} Δ H rxn delta, start text, H, end text, start subscript, start text, r, x, n, end text, end subscript, does not change when you add the enzyme. . This emphasizes a very important point ...
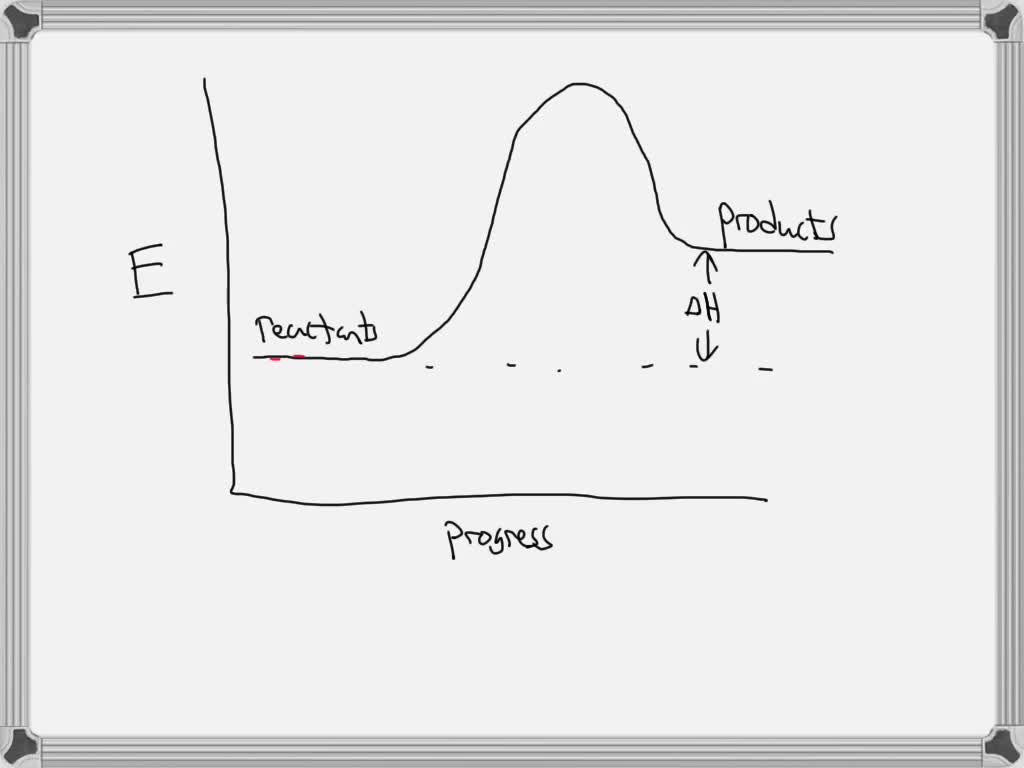
Solved Label the following reaction energy diagram for a - Chegg Question: Label the following reaction energy diagram for a catalyzed and an uncatalyzed process. AHx>0 AH 0 Eartwd) with catalyst Reaction Intermediate Earlwd) no catalyst Eatrev) with catalyst Potential energy Products Transition State Ea (rev) no catalyst Reactants Uncatalyzed Catalyzed Reaction progress Show transcribed image text Expert Answer

Label the following reaction energy diagram for a catalyzed and an uncatalyzed process.
Unit 5 Progress Check: FRQ - INVESTIGATING MATTER(S) Please respond on separate paper, following directions from your teacher. (b) On your energy diagram, draw a dashed curve to show the reaction progress from ... Date - ISD 622 Refer to the energy diagram below to answer the following questions. ... uncatalyzed reaction, and the other curve represents the energy profile for the ... Chapter 8 BIOCHEM Flashcards | Quizlet The graph presents three activation energy profiles for a chemical reaction (the hydrolysis of sucrose): an uncatalyzed reaction, and the same reaction catalyzed by two different enzymes. most product formed: rxn cat by enzyme B middle: reaction cat by enzyme Z Least product formed: uncatalyzed rxn factors that affect enzymes 1.
Label the following reaction energy diagram for a catalyzed and an uncatalyzed process.. Solved Label the following reaction energy diagram for a | Chegg.com Question: Label the following reaction energy diagram for a catalyzed and an uncatalyzed process. Arx > 0 Transition Stato Reactants Entwu no catalyst ... Enzymes and the active site (article) | Khan Academy WebReaction coordinate diagram showing the course of a reaction with and without a catalyst. With the catalyst, the activation energy is lower than without. However, the catalyst … Solved Label the following reaction energy diagram for a - Chegg WebLabel the following reaction energy diagram for a catalyzed and an uncatalyzed process. AHx>0 AH 0 Eartwd) with catalyst Reaction Intermediate Earlwd) no catalyst Eatrev) … Solved Label the following reaction energy diagram for … WebTranscribed image text: Label the following reaction energy diagram for a catalyzed and an uncatalyzed process. Transition State Transition State Transition intermediate Reaction Intermediate Transition State …
CHAPTER 17 The Reaction Process ... The following chemical equations describe these two reactions. ... Draw and label an energy diagram for a reaction in which E. Solved Label the following reaction energy diagram for … WebQuestion: Label the following reaction energy diagram for a catalyzed and an uncatalyzed process. Uncatalyzed Catalyzed Eare) with catalyst Ea {fwd) with catalyst 1 Products AHex > 0 -- - Potential energy … 12.7 Catalysis | Chemistry - Lumen Learning Example 1: Using Reaction Diagrams to Compare Catalyzed Reactions The two reaction diagrams below represent the same reaction: one without a catalyst and one with a catalyst. Identify which diagram suggests the presence of a catalyst, and determine the activation energy for the catalyzed reaction: Check Your Learning 18.4: Potential Energy Diagrams - Chemistry LibreTexts Web8 aug. 2022 · The energy changes that occur during a chemical reaction can be shown in a diagram called a potential energy diagram, or sometimes called a reaction progress …
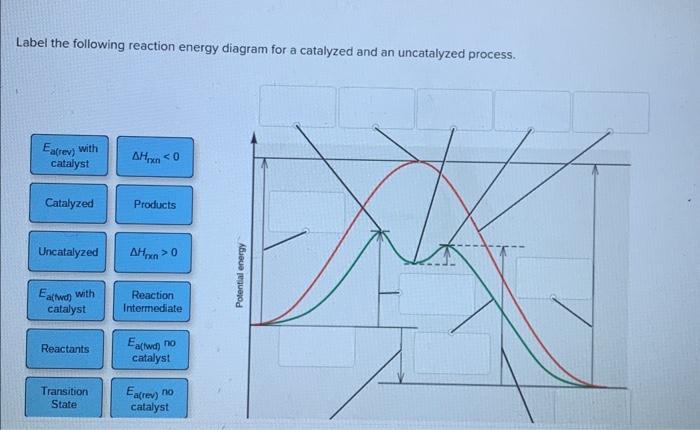
12.7 Catalysis - Chemistry 2e | OpenStax WebThe catalyzed reaction is the one with lesser activation energy, in this case represented by diagram b. Check Your Learning Reaction diagrams for a chemical process with and … Answered: Label the components of an energy… | bartleby ASK AN EXPERT. Science Chemistry Label the components of an energy diagram for a spontaneous reaction. Answer Bank uncatalyzed reaction products activation energy reactants catalyzed reaction Reaction progress-. Label the components of an energy diagram for a spontaneous reaction. Solved Label the following reaction energy diagram for a - Chegg WebQuestion: Label the following reaction energy diagram for a catalyzed and an uncatalyzed process. AHpXn > 0 Ealfwd) no catalyst Earrev) with catalyst Reactants … AP CHEMISTRY 2006 SCORING GUIDELINES the reaction. Diagram 1 represents a catalyzed pathway and diagram 2 represents an uncatalyzed pathway for the same reaction. One point is earned for identifying the correct graphs and indicating which represents which pathway. (ii) Indicate whether you agree or disagree with the statement in the box below. Support your answer with a short ...
Potential Energy Diagram of Catalyzed and Uncatalyzed Reactions Potential Energy Diagram of Catalyzed and Uncatalyzed Reactions Narcademy 2K subscribers Subscribe 3.9K views 2 years ago #narcademy Analyzing the potential energy diagram of a...
Scanned Document Potential Energy Diagrams. Answer the questions by referring to the diagram of the potential energy of a reaction. -ACTIVATEDUPLEX. Potential energy.
label the following reaction energy diagram for a catalyzed and an ... Take a look at this or that energy diagram. In the case of the uncatalyzed reaction, the more energy you have, the more energy you can save for the reaction. The catalyst is the element of life, zinc. Zinc is the fourth element in the periodic table. It is the element that enables life to occur.
12.7: Catalysis - Chemistry LibreTexts Oct 27, 2022 · A graph is shown with the label, “Reaction coordinate,” on the x-axis and the label,“Energy,” on the y-axis. Approximately half-way up the y-axis, a short portion of a black concave down curve which has a horizontal line extended from it across the graph.
Potential Energy Diagram of Catalyzed and Uncatalyzed Reactions Web29 mar. 2020 · 3.9K views 2 years ago #narcademy Analyzing the potential energy diagram of a regular/uncatalyzed and a catalyzed (adding a catalyst) reaction. Remember that the 🔼H of reaction …
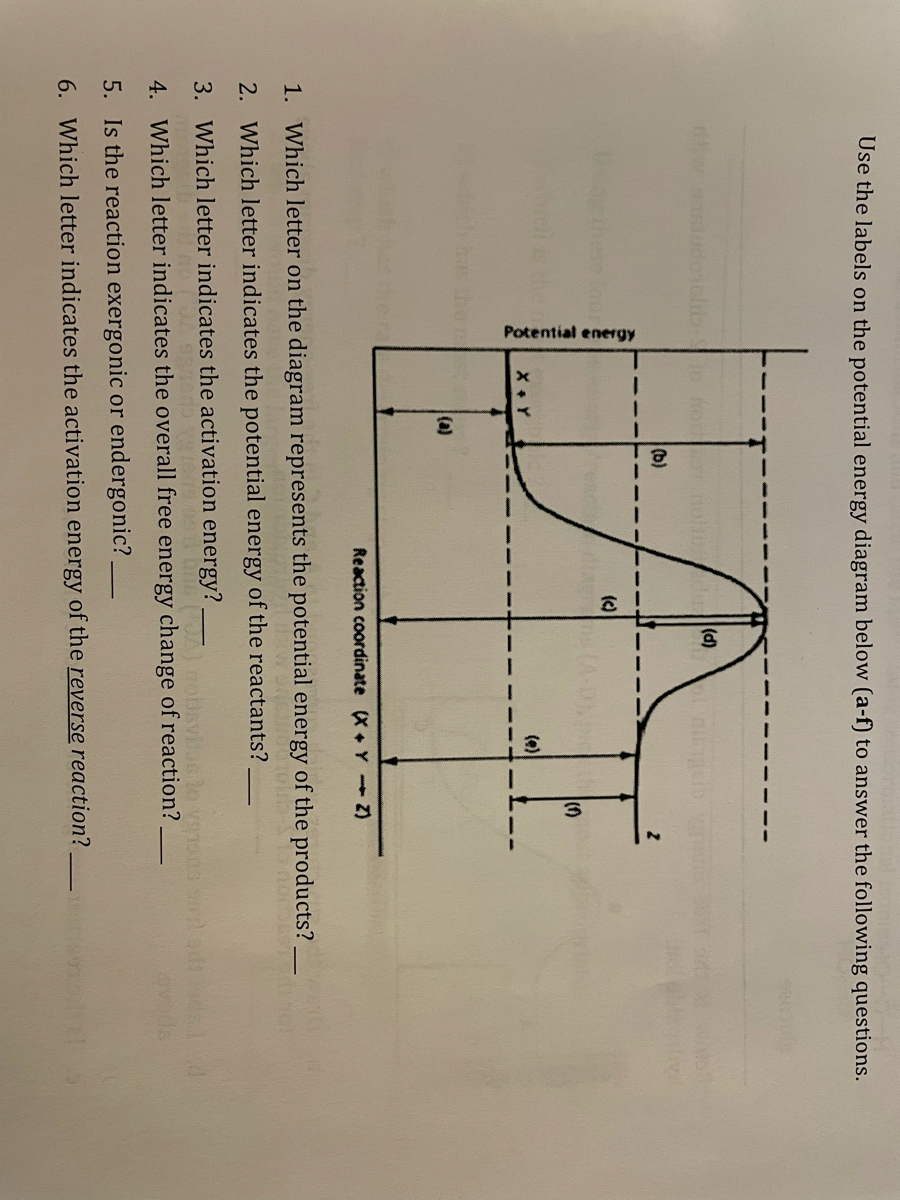
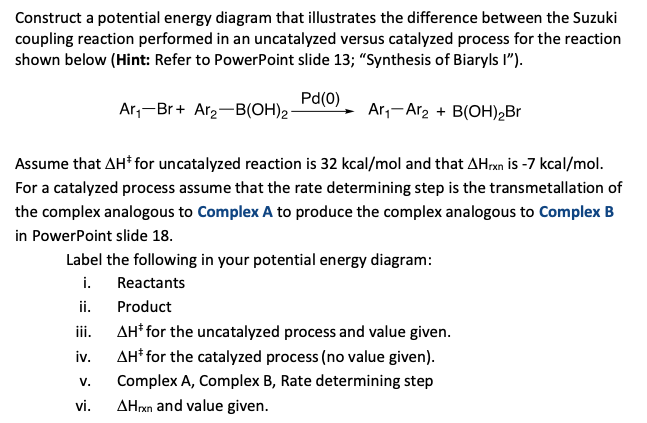
6.6: Reaction Coordinate Diagrams - Chemistry LibreTexts In an energy diagram, the vertical axis represents the overall energy of the reactants, while the horizontal axis is the ' reaction coordinate ', tracing from left to right the progress of the reaction from starting compounds to final products. The energy diagram for a typical one-step reaction might look like this:
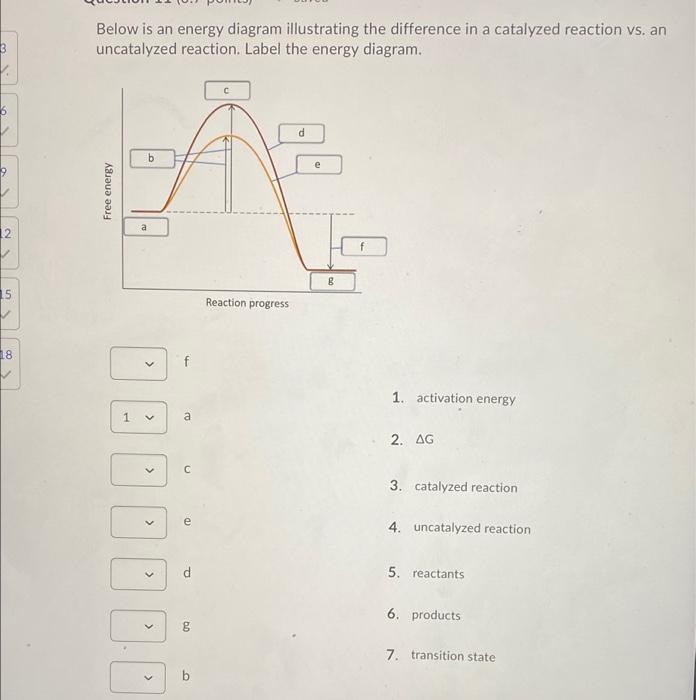
Answered: Macmillan Learning Label the components… | bartleby Transcribed Image Text: Macmillan Learning Label the components of an energy diagram for a spontaneous reaction (AG°<0). Energy Incorrect reactants standard free E of activation AG uncatalyzed reaction Reaction progress products catalyzed reaction ->>> Answer Bank
Solved Label the following reaction energy diagram for a - Chegg WebQuestion: Label the following reaction energy diagram for a catalyzed and an uncatalyzed process Transition State Reactants Products Catalyzed Uncatalyzed …
12.7 Catalysis - Chemistry 2e | OpenStax For the first step, Ea = 80 kJ for (a) and 70 kJ for (b), so diagram (b) depicts the catalyzed reaction. Activation energies for the second steps of both mechanisms are the same, 20 kJ. Homogeneous Catalysts A homogeneous catalyst is present in the same phase as the reactants.
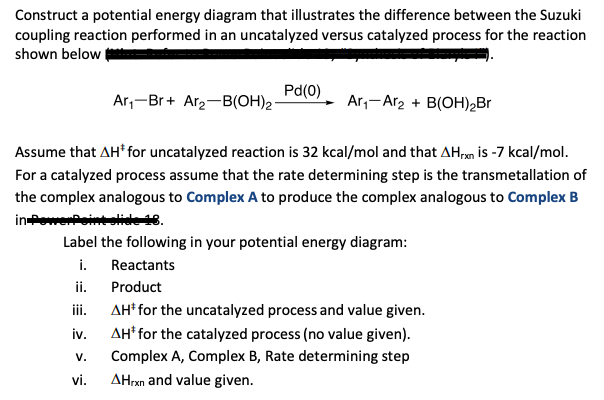
ap07 chemistry q6 - College Board The reaction is exothermic. The reaction is slow at 25°C; however, a catalyst will cause the reaction to proceed faster. (e) Using the axes provided below, draw the complete potential-energy diagram for both the catalyzed and uncatalyzed reactions. Clearly label the curve that represents the catalyzed reaction.
Solved Label the following reaction energy diagram for a ... Transition State Transition State Transition intermediate Reaction Intermediate Transition State Transition Uncatalyzed Transition State Reactants Products Catalyzed Ea (fwd) no catalyst Uncatalyzed AHxn < 0 Potential energy Ea (rev) no catalyst Ea (fwd) no catalyst Ea (rev) Show transcribed image text Expert Answer 100% (79 ratings)
Catalysis | Chemistry for Majors - Lumen Learning Reaction diagrams for an endothermic process in the absence (red curve) and presence (blue curve) of a catalyst. The catalyzed pathway involves a two-step mechanism (note the presence of two transition states) and an intermediate species (represented by the valley between the two transitions states).
18.4: Potential Energy Diagrams - Chemistry LibreTexts The energy changes that occur during a chemical reaction can be shown in a diagram called a potential energy diagram, or sometimes called a reaction progress curve. A potential energy diagramshows the change in potential energy of a system as reactants are converted into products.
Solved Label the following reaction energy diagram for a ... Question: Label the following reaction energy diagram for a catalyzed and an uncatalyzed process. help!! thx. Show transcribed image text. Best Answer. This is the best answer based on feedback and ratings. 88 % ...
Solved Label the following reaction energy diagram for a - Chegg WebTranscribed image text: Label the following reaction energy diagram for a catalyzed and an uncatalyzed process. Arx > 0 Transition Stato Reactants Entwu no catalyst Entwd) …
Drawing the Reaction Energy Diagram of a Catalyzed Reaction Example Problem 1 - Catalyzed Reaction Diagram. For the following simplified equation, label the reaction energy diagram with and without a catalyst. X ...
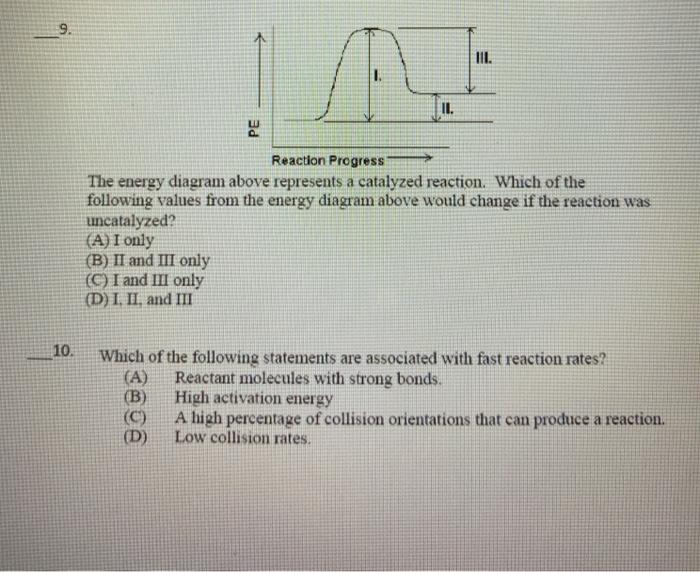
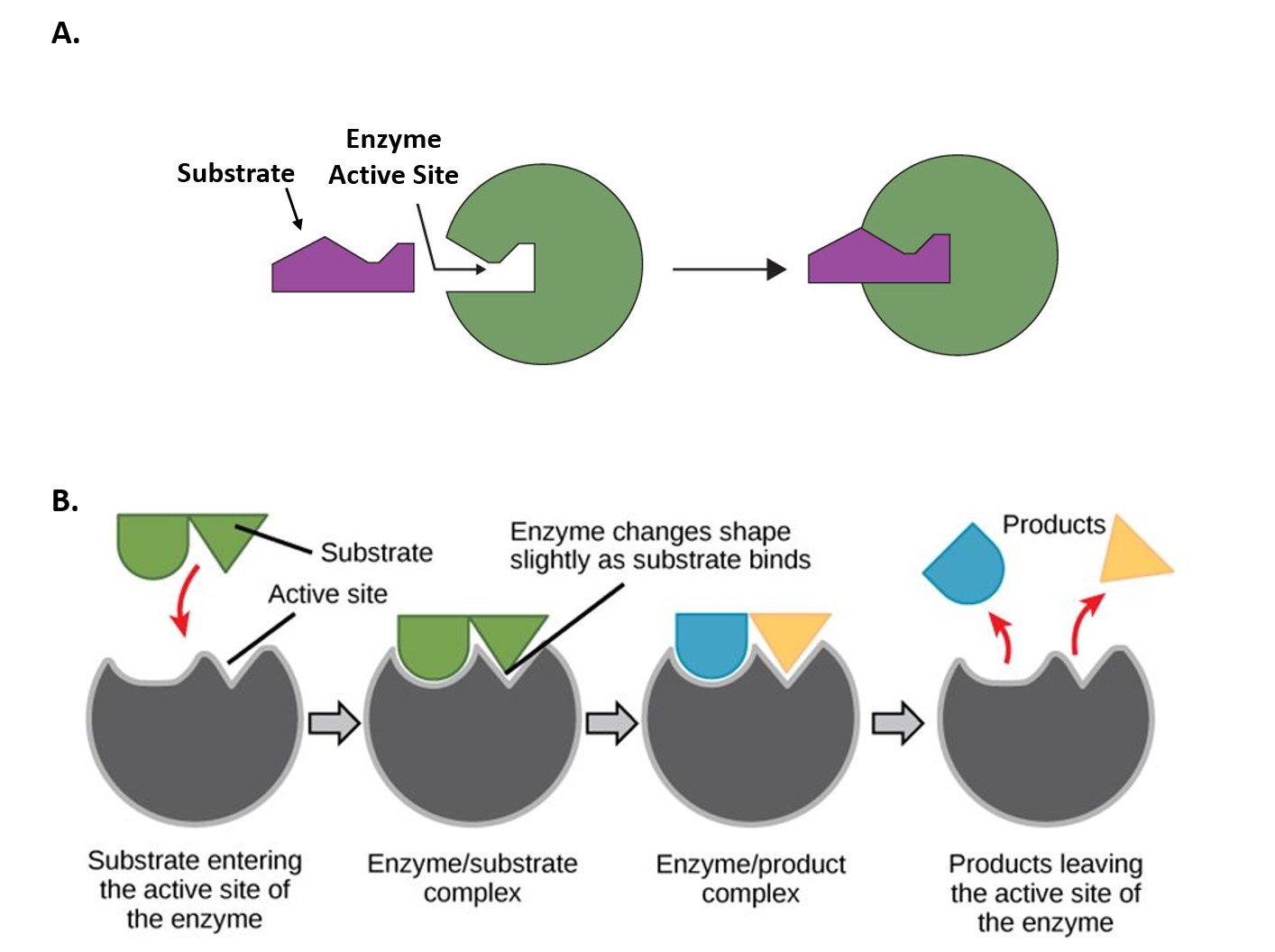
Chem Chapter 10 Homework 1 Flashcards | Quizlet The reaction energy diagrams for four reactions are given. Arrange the reactions according to their relative rates of reaction. Check all that apply The activation energy is higher in an uncatalyzed reaction than in an enzyme-catalyzed reaction. An enzyme-substrate complex (ES) is formed in an enzyme-catalyzed reaction.
Chapter 8 BIOCHEM Flashcards | Quizlet The graph presents three activation energy profiles for a chemical reaction (the hydrolysis of sucrose): an uncatalyzed reaction, and the same reaction catalyzed by two different enzymes. most product formed: rxn cat by enzyme B middle: reaction cat by enzyme Z Least product formed: uncatalyzed rxn factors that affect enzymes 1.
Date - ISD 622 Refer to the energy diagram below to answer the following questions. ... uncatalyzed reaction, and the other curve represents the energy profile for the ...
Unit 5 Progress Check: FRQ - INVESTIGATING MATTER(S) Please respond on separate paper, following directions from your teacher. (b) On your energy diagram, draw a dashed curve to show the reaction progress from ...





















Komentar
Posting Komentar