44 open label clinical trial
classic.clinicaltrials.gov › ct2 › showPROSPECTIVE OPEN LABEL CLINICAL TRIAL TO ADMINISTER A BOOSTER... Mar 15, 2022 · None (Open Label) Primary Purpose: Prevention: Official Title: PROSPECTIVE OPEN LABEL CLINICAL TRIAL TO ADMINISTER A BOOSTER DOSE OF PFIZER/BIONTECH OR MODERNA COVID-19 VACCINE IN HIGH-RISK INDIVIDUALS: Actual Study Start Date : July 30, 2021: Estimated Primary Completion Date : August 31, 2022: Estimated Study Completion Date : August 31, 2023 Phase 3, Open-label Clinical Trial of EB-101 for the Treatment of ... This open-label, controlled study will evaluate the efficacy and safety of EB-101 for the treatment of large, chronic, RDEB wounds. The study intervention consists of one-time surgical application of gene-corrected keratinocyte sheets (EB-101) for the treatment of RDEB wound sites in up to approximately 10-15 participants.
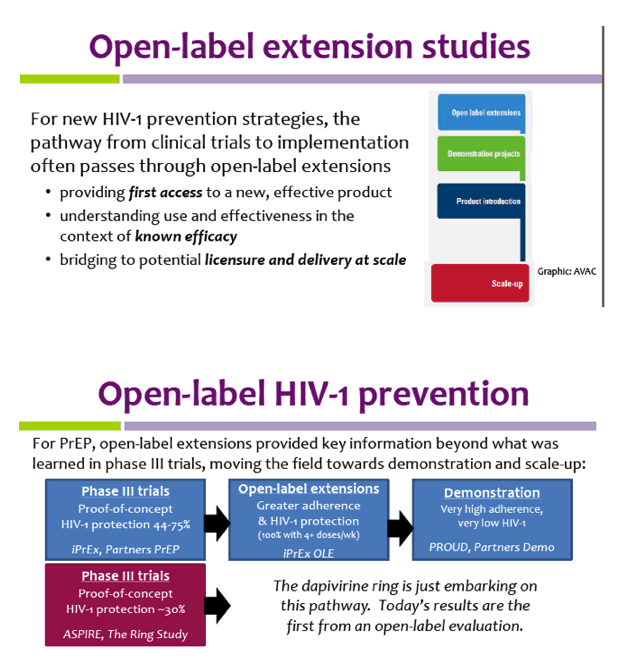
Open-label extension studies: do they provide meaningful ... - PubMed Most of the value accrued in open-label extension studies is gained from a refinement in the perception of the expected incidence of adverse effects that have most likely already been identified as part of the preclinical and clinical trial programme.

Open label clinical trial
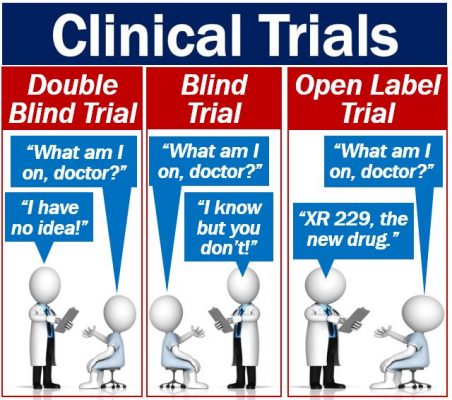
› understanding-clinical-trial-terminology-what-is-an-openUnderstanding Clinical Trial Terminology: What is an Open Label... Jun 24, 2019 · Open-label trials can be used to compare treatments or gather additional information about the long-term effects in the intended patient population. In some instances, patients who complete one clinical trial may be eligible to continue in an open-label extension study where all participants are eligible to receive active treatment for an ... clinicaltrials.gov › ct2 › showBaricitinib, Methotrexate as Monotherapy or Combination in the... Apr 25, 2023 · This open-label randomized clinical trial will be conducted in the department of rheumatology, BSMMU. The rheumatoid arthritis patients with moderate to high disease activity at baseline, disease activity score (DAS28ESR>3.2) will be considered as primary entry criteria for this study. Consecutive sampling method will be applied. en.wikipedia.org › wiki › Open-label_trialOpen-label trial - Wikipedia An open-label trial, or open trial, is a type of clinical trial in which information is not withheld from trial participants. [1] In particular, both the researchers and participants know which treatment is being administered. [1] This contrasts with a double-blinded trial, where information is withheld both from the researchers and the ...
Open label clinical trial. Effects of open-label placebos in clinical trials: a ... - Nature Placebos have been the subject of many studies in the last two decades 1 and the number of clinical trials to examine a placebo treatment as the primary intervention is rapidly growing 2.Research ... The Oral PI3Kδ Inhibitor Linperlisib for the Treatment of Relapsed and ... A phase II, single-arm, open-label clinical trial enrolled 84 patients from 25 sites in China from April 2019 to September 2020, with an analysis cutoff date of September 30, 2021. The list of participating centers is shown in Supplementary Table S1. FDA Guidance: "Design Considerations for Pivotal Clinical ... Clinical Outcome Study Designs Double-masked (blinded), randomized, controlled, multi-center clinical trials (RCTs) are the "gold standard" for clinical outcome studies (but not necessarily... ascopubs.org › doi › absPostoperative Adjuvant Anastrozole for 10 or 5 Years in Patients... Apr 20, 2023 · PURPOSE Treatment with an aromatase inhibitor for 5 years is the standard treatment for postmenopausal hormone receptor–positive breast cancer. We investigated the effects of extending this treatment to 10 years on disease-free survival (DFS). PATIENTS AND METHODS This prospective, randomized, multicenter open-label phase III study assessed the effect of extending anastrozole treatment for ...
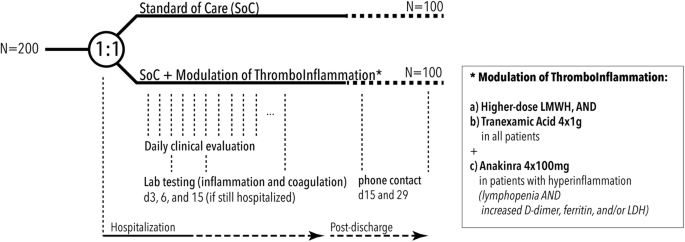
clinicaltrials.gov › ct2 › showDose Escalation, Open-Label Clinical Trial to Evaluate Safety ... Jun 1, 2022 · This Phase I, dose escalation, open label clinical trial is the first study of mRNA-1215 in healthy adults to evaluate the safety, tolerability, and immunogenicity of a Nipah virus (NiV) mRNA vaccine. The hypotheses are that the vaccine will be safe, tolerable, and will elicit an immune response in healthy adults. Study Product: Preventing COVID-19 Complications With Low- and High-dose ... Preventing COVID-19-associated Thrombosis, Coagulopathy and Mortality With Low- and High-dose Anticoagulation: a Multicentric Randomized, Open-label Clinical Trial: Actual Study Start Date : April 28, 2020: Actual Primary Completion Date : June 2, 2021: Actual Study Completion Date : June 2, 2021 NIH Clinical Center Search the Studies: Study Number, Study Title VRC 322/DMID 21-0016: A Phase I, Dose Escalation, Open-Label Clinical Trial to Evaluate Safety, Tolerability and Immunogenicity of a Nipah Virus (NiV) mRNA Vaccine, mRNA-1215, in Healthy Adults 000609-I: Pilot Study to Assess the Use of JSP191 in Matched Unrelated Donor Transplantation for Chronic Granulomatous Disease (CGD) 000410-I: Guidance for Clinical Trial Sponsors - Food and Drug Administration A clinical trial DMC is a group of individuals with pertinent expertise that reviews on a regular basis accumulating data from one or more ongoing clinical trials. The DMC advises the sponsor
Tucidinostat and Metronomic Capecitabine for Metastatic Triple-negative ... None (Open Label) Primary Purpose: Treatment: Official Title: Tucidinostat and Metronomic Capecitabine for Metastatic Triple-negative Breast Cancer:a Multicenter,Open-label, Randomized Controlled, Phase II Clinical Trial: Actual Study Start Date : August 1, 2022: Estimated Primary Completion Date : February 1, 2024: Estimated Study Completion ... Open-Label Trial - an overview | ScienceDirect Topics Open-label trials of desipramine, tranylcypromine, reboxetine, and bupropion showed improvement with the drug therapy.55 An open-label trial of escitalopram for SAD patients (N = 20) over 8 weeks produced a response rate of 95% (SIGH-SAD < 50% of baseline value) and a remission rate (SIGH-SAD score < 8) of 85%. 56 In an open-label trial of … Reducing bias in open-label trials where blinded outcome ... - PubMed Background: Blinded outcome assessment is recommended in open-label trials to reduce bias, however it is not always feasible. It is therefore important to find other means of reducing bias in these scenarios. Methods: We describe two randomised trials where blinded outcome assessment was not possible, and discuss the strategies used to reduce the possibility of bias. Placebos and Blinding in Randomized Controlled Cancer Clinical Trials ... In open-label comparative trials for which investigator bias may be of concern, a blinded central independent review of scans may mitigate bias regarding endpoint assessment. 3.
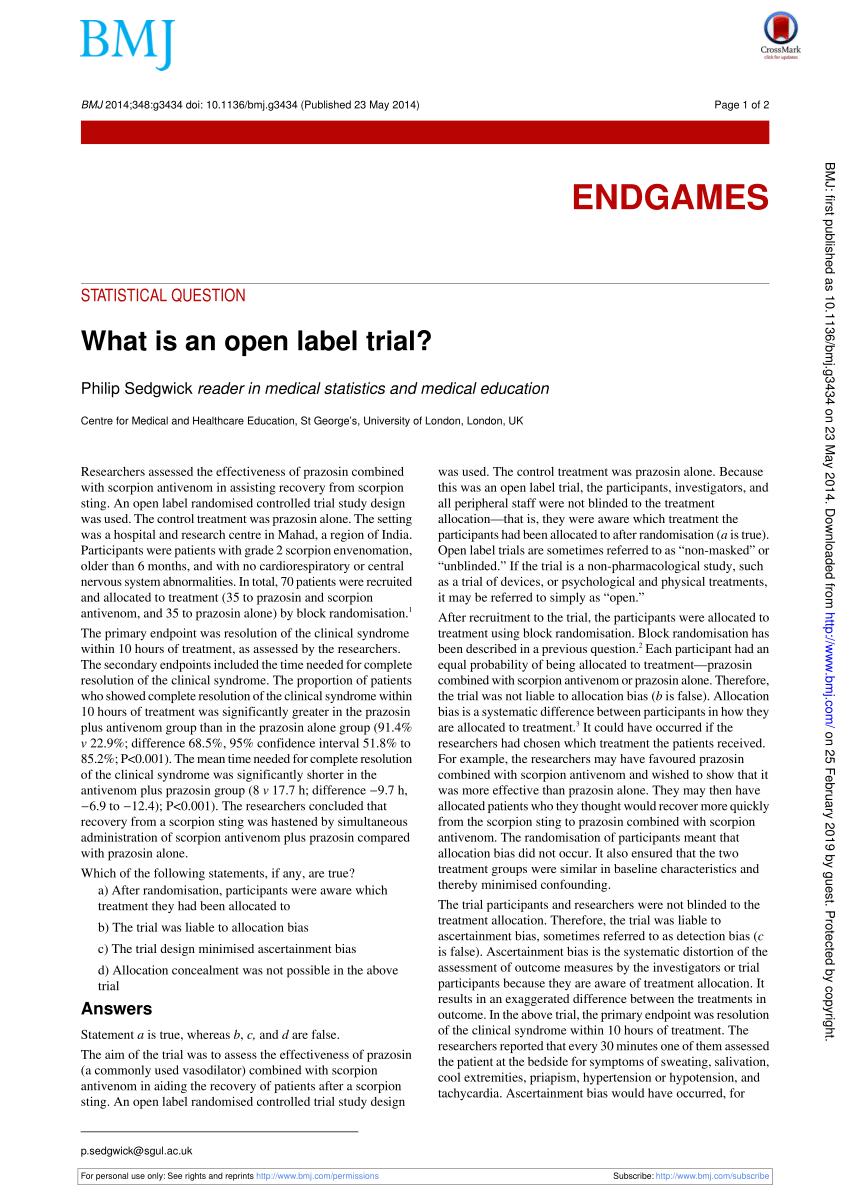
What is an open label trial? | The BMJ An open label randomised controlled trial study design was used. The control treatment was prazosin alone. The setting was a hospital and research centre in Mahad, a region of India. Participants were patients with grade 2 scorpion envenomation, older than 6 months, and with no cardiorespiratory or central nervous system abnormalities.
Open-Label Trial | NIH - HIV.gov Open-Label Trial A type of clinical trial. In open-label trials, both the researchers and participants know which drug (or other intervention) is being given to participants. Related Term (s) Clinical Trial Double-Blind Study
› guides › what-is-an-open-label-study-in-clinical-researchWhat is an Open Label Study in Clinical Research? | Power In an open label study, or open label trial, all participants know exactly what treatment/intervention they will be receiving in the study. Study physicians and investigators also know what each participant is receiving. That means that there is no uncertainty regarding whether or not you may be receiving a placebo rather than the study treatment.
› articles › s41598/023/32515-6A randomized, open-label clinical trial in mild cognitive... Apr 3, 2023 · Study design. The ACE-2020-EGb 761 study is a Phase IV, single-center, randomized, open-label, parallel-group clinical trial (CT) performed at the Ace Alzheimer Center Barcelona.
A roadmap to using randomization in clinical trials The study includes three periods: an open-label single-arm active treatment for 28 weeks to identify treatment responders (Period 1), a 24-week randomized treatment withdrawal period to primarily assess the efficacy of the active treatment vs. placebo (Period 2), and a 3-year long-term safety, open-label active treatment (Period 3).
› life-sciences › What-is-an-Open-Label-Clinical-TrialWhat is an Open-Label Clinical Trial? - News-Medical.net Mar 31, 2022 · Open-label trials can be used to gather additional safety and efficacious data on drugs on the market to increase the confidence of clinicians, patients, and clinical bodies. They can play...
Open-label placebo clinical trials: is it the rationale, the ... Clinical trials into open-label placebos (OPL) appear to show promise for a range of self-reported conditions but have been hampered by small sample sizes and short duration. What are the new findings? Placebo concepts refer to: (1) 'methodological controls'; or (2) 'mind-body treatment interventions'. ...
dian.wustl.edu › our-research › clinical-trialEnd of Trial and Open-Label Extension (OLE) Frequently Asked... End of Trial and Open-Label Extension (OLE) Frequently Asked Questions (FAQ) When did the solanezumab ("sola") and gantenerumab ("gant") blinded drug arms in the DIAN-TU trial officially end? When did the DIAN-TU announce the results of the current trial drug arms of solanezumab and gantenerumab; and where can I look for the results?
en.wikipedia.org › wiki › Open-label_trialOpen-label trial - Wikipedia An open-label trial, or open trial, is a type of clinical trial in which information is not withheld from trial participants. [1] In particular, both the researchers and participants know which treatment is being administered. [1] This contrasts with a double-blinded trial, where information is withheld both from the researchers and the ...
clinicaltrials.gov › ct2 › showBaricitinib, Methotrexate as Monotherapy or Combination in the... Apr 25, 2023 · This open-label randomized clinical trial will be conducted in the department of rheumatology, BSMMU. The rheumatoid arthritis patients with moderate to high disease activity at baseline, disease activity score (DAS28ESR>3.2) will be considered as primary entry criteria for this study. Consecutive sampling method will be applied.
› understanding-clinical-trial-terminology-what-is-an-openUnderstanding Clinical Trial Terminology: What is an Open Label... Jun 24, 2019 · Open-label trials can be used to compare treatments or gather additional information about the long-term effects in the intended patient population. In some instances, patients who complete one clinical trial may be eligible to continue in an open-label extension study where all participants are eligible to receive active treatment for an ...





























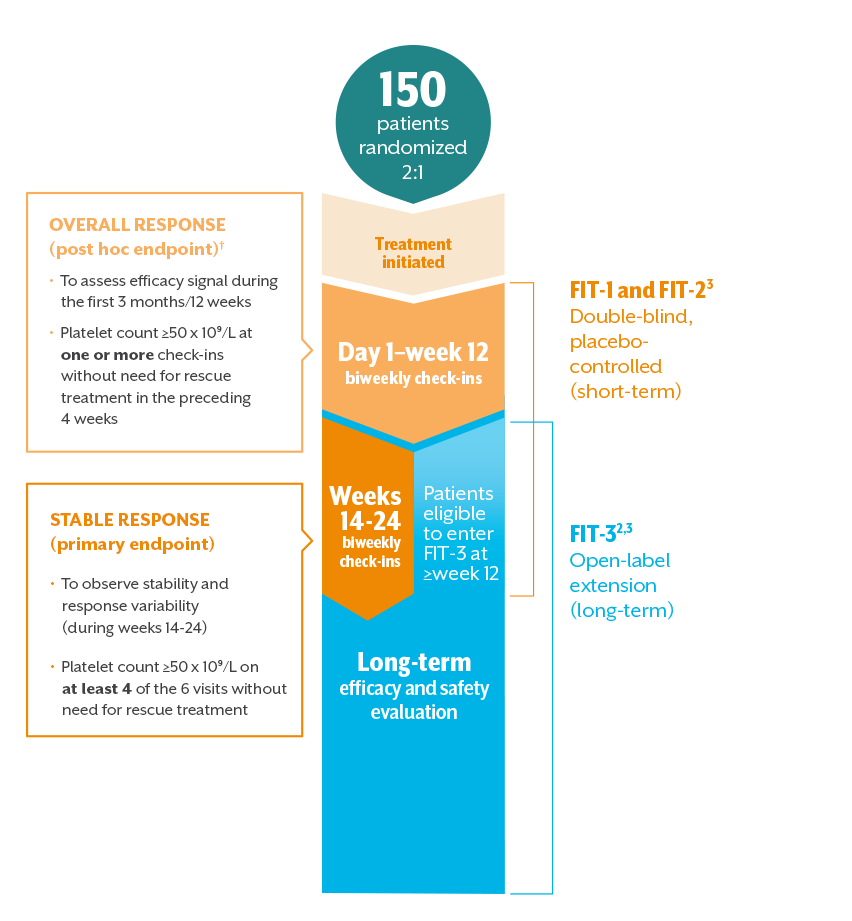


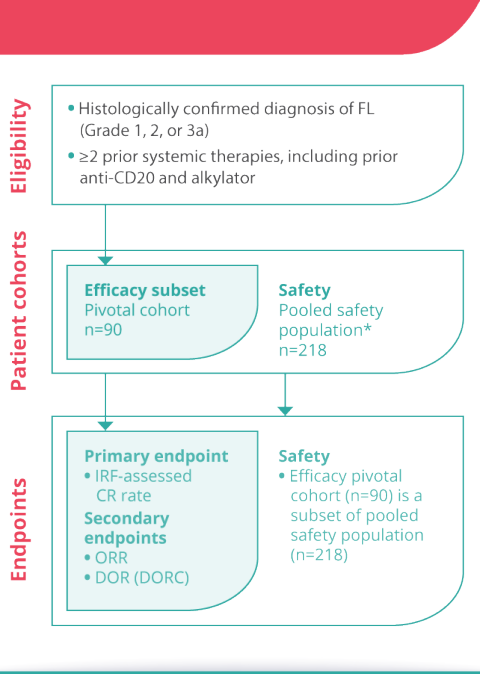
Komentar
Posting Komentar